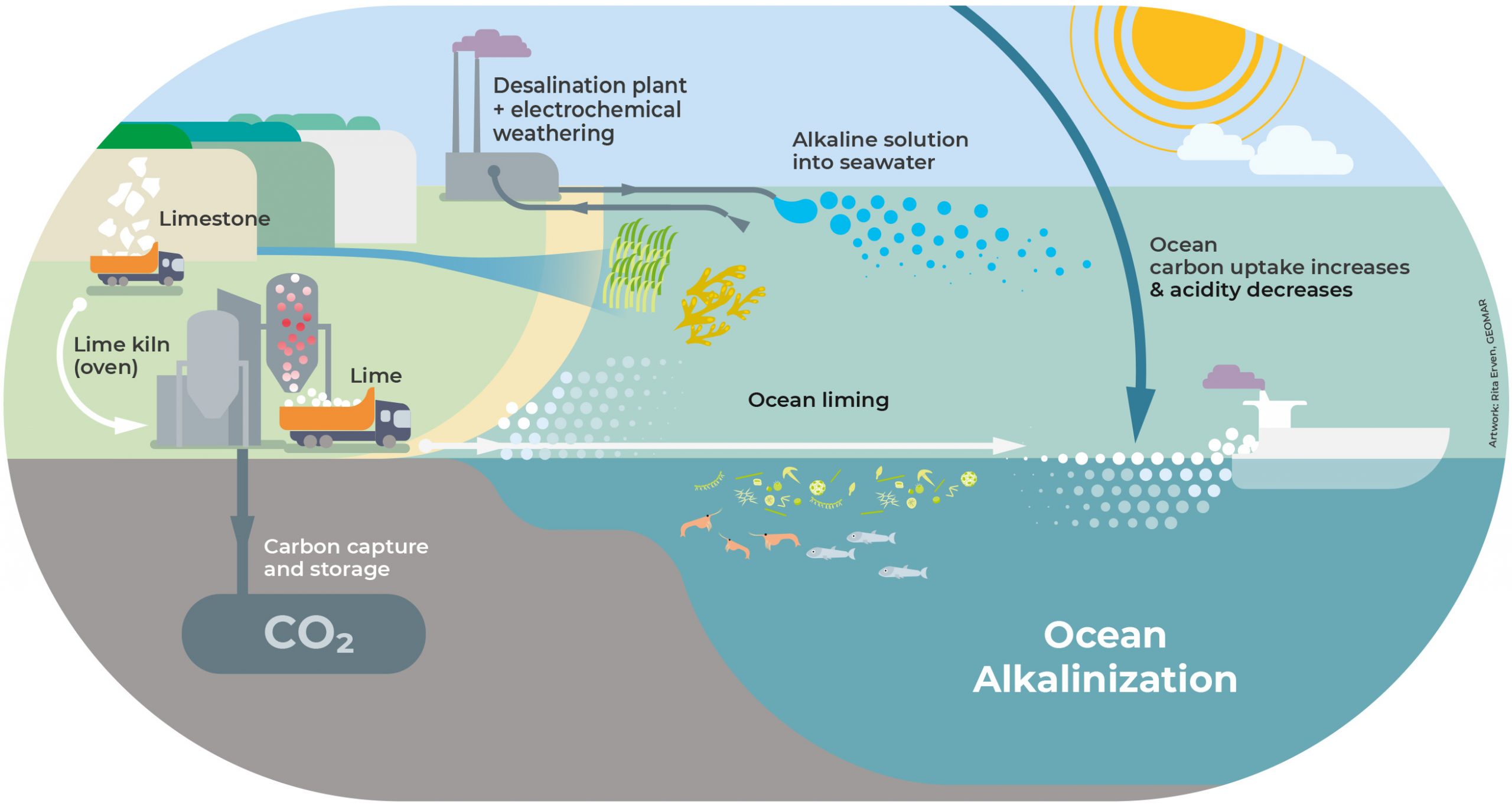
Alkalinity is the capacity of a solution to neutralize acid. By increasing the alkalinity of the upper ocean, the carbon storage capacity of seawater can be enhanced and thus, more CO2 can be taken up by the ocean.
Ocean Alkalinization approaches involve the addition of carbonate-containing minerals or alkaline solutions to the ocean, which then react with CO2 and water to form bicarbonate (HCO3–) and carbonate (CO32-) ions:
1) Alkaline rocks such as limestone can be mined to create lime, which could then be added directly to the ocean as mineral powder (ocean liming).
2) Another suggested approach is to react seawater with alkaline minerals through electrolysis in a full cell (e.g. desalination plant) before releasing the alkaline solution back into the ocean.
Adding alkalinity to the ocean would not only increase CO2 uptake by the ocean, but it would also counter seawater acidity generated by excess anthropogenic CO2. Considering that mineral weathering and ocean alkalinity production are the primary mechanisms whereby nature is consuming and storing CO2, investigating this approach seems worthwhile.
